Webinar on March 5
On March 5 at 2 pm ET, Med Safety Board (MSB) (a subsidiary of the Institute for Safe Medication Practices) will host an educational program in which expert faculty will review therapy considerations for reducing the risk of cisplatin-induced ototoxicity in pediatric, adolescent, and young adult patients.
Cisplatin is a commonly used chemotherapeutic agent for the treatment of certain childhood cancers, including germ cell tumors, hepatoblastoma, medulloblastoma, neuroblastoma, and osteosarcoma. However, as a known ototoxic agent, patients can risk permanent, bilateral hearing loss due to cisplatin use, with pediatric patients, especially younger children, at an increased risk for severe ototoxic effects. Hearing loss in this patient population can lead to delays in speech and language development and can also have a detrimental psychosocial and quality-of-life impact on pediatric, adolescent, and young adult patients receiving cisplatin therapy.
This educational program will discuss the incidence and mechanism of cisplatin-induced ototoxicity. Expert faculty will share safety and efficacy data of sodium thiosulfate injection in the prevention of cisplatin-induced ototoxicity. The safety implications of select inactive ingredients in pharmaceutical products in the pediatric population will also be addressed.

Take Our Survey
Have you or your child been treated with cisplatin? ACCO wants to hear about your experiences!
Click the button below to take our short survey and give us feedback on when the treatment took place and if there was medication involved to mitigate hearing loss.
TAKE THE SURVEYFighting cancer is hard enough without worrying about your child’s hearing, too.
What is Ototoxicity?
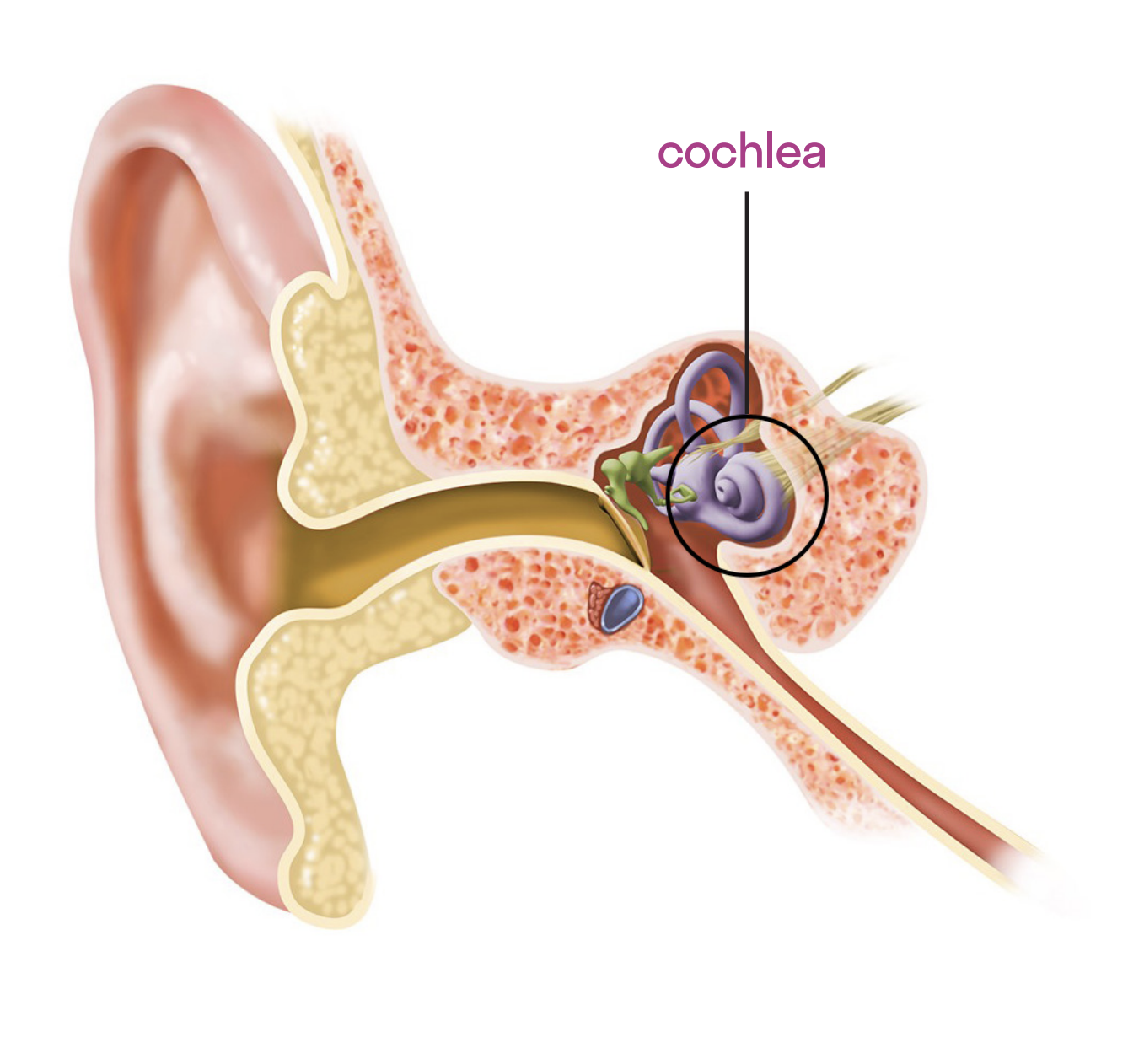
The 5-year survival rate for childhood cancers due to major treatment advances is now 85% or higher. Cisplatin-based therapy is an indispensable component of treatment for pediatric solid-tumor cancers; however, an unfortunate side effect of cisplatin-based chemotherapy is irreversible ototoxicity (or hearing loss). Ototoxicity is caused by irreversible damage to hair cells in the cochlea. This damage is generally dose-dependent, bilateral (affecting both ears), and can be progressive. From the very first treatment cycle, pediatric patients can suffer cisplatin-induced ototoxicity that may progressively worsen even after treatment ends.
Hearing loss can be seen in ~60 percent of children treated with cisplatin and can be as high as 90 percent.
How is it Treated?
Previously, intervention only occurred after hearing loss had been detected, and does not return normal hearing. The most common management strategy is the use of lifelong hearing aids, which do not completely reverse hearing loss and require replacements every 3-5 years and may also require amplification technology.4,7 Some children receive difficult to manage cochlear (inner ear) implants, which remain suboptimal in the direct recovery of hearing function and may also require replacement during an individual’s lifetime.
New Preventative Hearing Loss Treatment
PEDMARK is the first and only Food & Drug Administration (FDA)-approved treatment specifically designed to protect your child’s ears after cisplatin treatment.
It isn’t known exactly how PEDMARK works, but scientists think it may:
- Help the body make antioxidants that fight the toxic build-up caused by cisplatin
- Neutralize the toxins made by cisplatin molecules
- Attach to the cisplatin molecules to reduce the amount of toxins they releaseConsult with your child’s medical professional about the possible benefits of PEDMARK.Click here to see references.



